
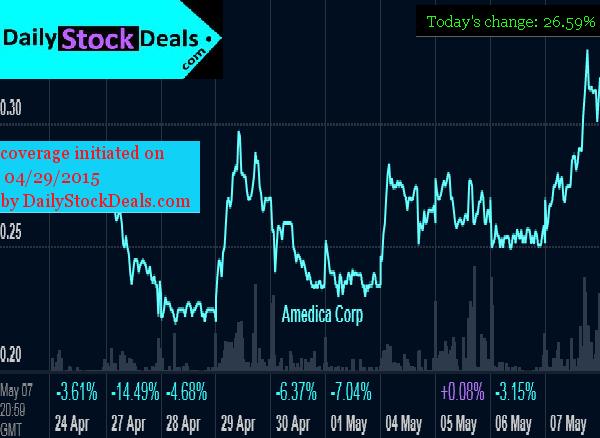
Amedica Corporation, AMDA, Profile, Summary
Amedica Corporation, AMDA, is a commercial biomaterial company. The Company is focused on using its silicon nitride technology platform to develop, manufacture and sell a range of medical devices. The Company markets spinal fusion products and are developing products for use in total hip and knee joint replacements.
Biomaterials are synthetic or natural materials available in a variety of forms that are used in virtually every medical specialty. The Company markets its Valeo family of silicon nitride interbody spinal fusion devices in the United States and Europe for use in the cervical and thoracolumbar areas of the spine. In addition, to its silicon nitride-based spinal fusion products, it markets a complementary line of non-silicon nitride spinal fusion products which allows providing surgeons and hospitals with a range of products. The Company produces its silicon nitride advanced ceramic in four forms: a fully dense, load-bearing solid, referred to as MC2; a porous bone-like cancellous structured form, referred to as CSC; a composite incorporating both its solid MC2 material and its porous CSC material intended to promote an environment for bone growth; and a coating for application onto other biomaterials.
The Company competes with Medtronic, Inc.; DePuy Synthes Companies, a group of Johnson & Johnson companies; Stryker Corporation; Biomet, Inc.; Zimmer Holdings, Inc.; Smith & Nephew plc; Aesculap Inc; CeramTec, Kyocera, and CoorTek, Inc.
OSTEOPROMOTIVE: DELIVERS ENHANCED OSTEOGENIC RESPONSE
-
Enhanced osteopromotion: The surface chemistry and nanostructure topography of Si3N4 provides an optimal environment for stimulation of osteoprogenitor cells to differentiate into osteoblasts.
-
Greater protein adsorption: Si3N4 demonstrates significantly greater protein adsorption (fibronectin, laminin and vitronectin) in comparison to PEEK and titanium. (1)
-
Greater new bone formation: Si3N4 implants demonstrate greater new bone formation at 3, 7, 14 and 90 days compared to PEEK and titanium in in vivo study; regenerated bone associated with Si3N4 implants is 2 to 3 times that of PEEK and titanium implants at 3 months after surgery.(2)
-
Increased osteointegration: Si3N4 implants demonstrate increased osteointegration at 3, 7, 14 and 90 days compared to PEEK and titanium in in vivo studies; percent of bone at Si3N4 implant interface is 2 to 6 times that of PEEK and titanium implants at 3 months after surgery.(2)
DEMONSTRATED ANTI-INFECTIVE PROPERTIES
-
Superior antibacterial function: Si3N4 inhibits biofilm formation and bacterial colonization. Si3N4 demonstrates significantly lower biofilm formation at 4, 24, 48 and 72 hours as compared to PEEK and titanium; live bacteria (S. epidermidis, S. aureus, P. aeruginosa, E. coli and Enterococcus) associated with Si3N4 implants are 8 to 30 times lower than PEEK and titanium.(1)
-
Demonstrated bacteriostatic agent: In in vivo studies, no infection is observed with bacteria-inoculated Si3N4 implants at 3 months, whereas both PEEK and titanium implants maintain a septic state. Si3N4 demonstrates this property even in the absence of antibiotics.(2)
EXCELLENT IMAGING PROPERTIES
-
Compatible with all imaging modalities: Si3N4 implants are semi-radiolucent with clearly visible boundaries, and produce no distortion under MRI and no scattering under CT; this enables an exact view of the implant for precise intraoperative placement and postoperative fusion assessment.
Sources: The Company, OxBridge Research, OTCKING, DailyStockDeals, OTCstockIQ
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
If you would like your company featured or want to learn more, please don't hesitate to contact the Editor. editor [@] OxBridgeResearch.com





















