
Delcath Systems, Inc. is a specialty pharmaceutical and medical device company focused on oncology. Our proprietary product-Melphalan Hydrochloride for Injection for use with the Delcath Hepatic Delivery System (Melphalan/HDS)-is designed to administer high dose chemotherapy to the liver, while controlling the systemic exposure to those agents. The Company's principal focus is on the treatment of primary and metastatic liver cancers. In the United States, the Melphalan/HDS system is considered a combination drug and device product, and is regulated as a drug by the United States Food and Drug Administration (FDA). The Melphalan/HDS system has not been approved for sale in the United States. In Europe, our proprietary system to deliver and filter melphalan hydrochloride is marketed as a device under the trade name Delcath Hepatic CHEMOSAT® Delivery System for Melphalan (CHEMOSAT). In April 2012, we obtained authorization to affix a CE Mark for the Generation Two CHEMOSAT system. The right to affix the CE mark allows the Company to market and sell the CHEMOSAT system in Europe. The Company has commenced a global phase 2 clinical trial in Europe to investigate Melphalan/HDS system for primary liver cancer and is initiating plans to evaluate intrahepatic cholangiocarcinoma.
Cancers in the Liver
According to the American Cancer Society, cancers in the liver are among the most prevalent and lethal forms of the disease. They can either be primary, originating in the liver, or metastatic, originating elsewhere and traveling to the liver, creating new tumors there. The current treatment armamentarium for cancers in the liver includes surgery, transplantation, systemic chemotherapy, a variety of focal and regional therapies, and radiation therapy, which have varying degrees of invasiveness, efficacy, and side effects.
Developing liver-directed high dose chemotherapy
Delcath is developing a proprietary system for liver-directed high dose chemotherapy. Years of clinical research have gone into refining this system.
Delcath’s proprietary system for liver-directed high dose chemotherapy utilizes the chemotherapeutic agent melphalan hydrochloride at a dose of 3.0 mg/kg, a dose selected based on a Phase 1 clinical trial conducted at the National Cancer Institute in patients with hepatic malignancies. More recently a Phase 3 study compared Delcath’s system for liver directed high dose chemotherapy with melphalan to best alternative care (BAC) for patients with metastatic cancer in the liver caused by ocular and cutaneous melanoma. Data from this trial is pending publication.
Additionally, a recently completed phase 2 clinical trial evaluated liver directed high dose chemotherapy with melphalan in four different tumor types: primary liver cancer, metastatic neuroendocrine tumors (NET), adenocarcinoma, and melanoma. Results of the NET arm were presented at ESMO 2011 (link to bibliography slides). Data from this trial is pending publication.
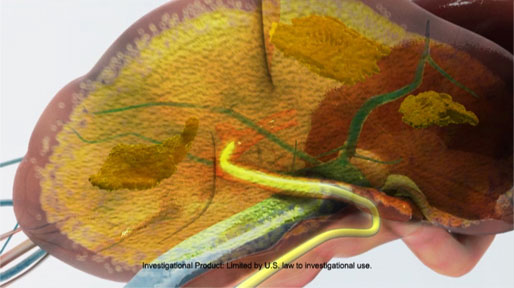
What Is Liver-Directed High Dose Chemotherapy?
Liver-directed high dose chemotherapy evolved from a highly invasive open surgical procedure to a minimally invasive procedure known as percutaneous hepatic perfusion (PHP). During PHP, three catheters are placed percutaneously through standard interventional radiology techniques. The liver is temporarily isolated from the body’s circulatory system, during which time a 30 minute infusion of the chemotherapeutic agent melphalan hydrochloride directly to the liver occurs. The blood is collected as it exits the liver for filtration by proprietary filters prior to returning it to the patient.
In the United States, Delcath’s system for PHP has been evaluated in a Phase 3 trial for patients with unresectable ocular and cutaneous liver metastases. Additional cancers including colorectal cancer, neuroendocrine tumors and in patients with hepatocellular carcinoma (primary liver cancer) have been studied. These studies are presently being evaluated by the FDA.
How Does the Technology Work?
Liver-directed high dose chemotherapy via percutaneous hepatic perfusion involves three basic steps: 1) Isolation2) Infusion and 3) Hemofiltration.
1. Isolation
First, an infusion catheter is inserted into the femoral artery and guided so that the tip of the catheter is within the hepatic artery to deliver the anticancer drug, melphalan hydrochloride. Next, the Delcath isolation-aspiration catheter is inserted into the femoral vein and guided into the inferior vena cava. In the inferior vena cava, two occlusion balloons of the isolation-aspiration catheter are inflated to block the normal venous outflow of blood from the liver to the heart, thereby isolating the liver.
2. Infusion
High doses of melphalan are delivered directly to the liver via the infusion catheter over a period of 30 minutes, saturating the liver and the tumor tissue.
3. Hemofiltration
The isolation-aspiration catheter collects the blood as it exits the liver in the region between the two inflated balloons and then directs it out of the body. The blood then passes through the Delcath proprietary hemofiltration system, which reduces the concentration of chemotherapeutic agent. The filtered blood can now be returned to the patient's body through a third catheter placed in the internal jugular vein.
Source: The Company, OxBridge Research, Daily Stock Deals, OTC Stock Wire
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!® Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
If you want to get your company featured on Daily Stock Deal or want to learn more, please contact the Editor. Thanks you!





















