
VBI Vaccines, VBIV, Company Profile
VBI Vaccines is a commercial-stage biopharmaceutical company developing a next generation of vaccines to address unmet needs in infectious disease and immuno-oncology. VBI is advancing the prevention and treatment of hepatitis B and recently completed its Phase 3 program in the U.S., Europe, and Canada. VBI is headquartered in Cambridge, MA.
The company recently announced data from three preclinical mouse studies conducted to enable selection for the company’s coronavirus program, VBI-2900. As a result of these studies, VBI has selected two vaccine candidates, with the potential to be one-dose vaccines, to take into an adaptive Phase 1/2 human clinical study, expected to begin around year-end 2020, subject to regulatory approval: (1) VBI-2901 and (2) VBI-2902, vaccine candidate for (COVID-19) spike protein.
VBI also entered into an agreement with Therapure Biomanufacturing, an integrated Contract Development and Manufacturing Organization (CDMO), for development and manufacturing services in preparation for production of its coronavirus vaccine candidates. The collaboration with Therapure is expected to enable the initiation of clinical studies by the end of 2020. As part of the agreement, Therapure will manufacture bulk vaccine for use through Phase 2 clinical studies.
THE BOTTOMLINE: two major things are expected to happen by Dec 2020
1. The Company is seeking FDA approval for Hepatitis B Vaccine (successfully completed phase 3)
2. Planning to start preclinical studies of Corona Virus Vaccine (Phase 1) if approved by the government.
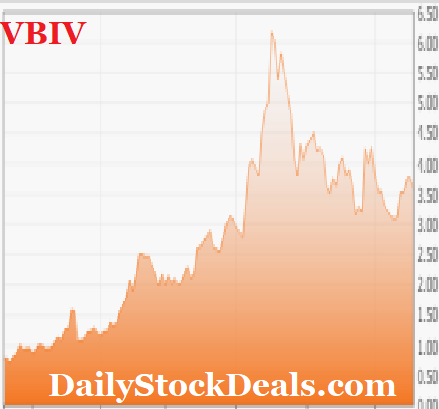
A major Brokerage Firm has recently upgraded the stock with a price target of $9
Platform Overview
VBI Platform allows for the design of enveloped (“e”) virus-like particle vaccines (eVLPs) are an innovative new class of synthetic vaccines that are designed to closely mimic the structure of viruses.
Because of their structural similarity to viruses found in nature, vaccination with a target protein expressed in an eVLP is capable of imparting greater immunity than vaccination with the same recombinant target protein alone.
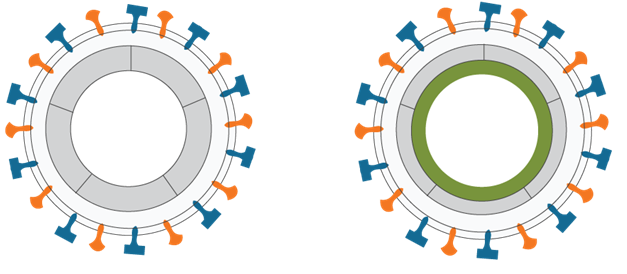
Bivalent–Multiple Surface Proteins Trivalent – Internal Protein
eVLP Advantages
· Highly Immunogenic: Immune responses comparable to or better than natural infection by closely mimicking structure of target virus.4
· Customizable: Ability to rationally design a vaccine by including different antigens and controlling their relative expression.
· Safe: Unlike live-attenuated vaccines, VLPs cannot revert back to an infectious state.
· Commercially Viable: Manufactured and purified using scalable methods; demonstrated high yields and purity.5
eVLPs are highly customizable, which allows VBI to rationally design preventative or therapeutic vaccine candidates by controlling the expression of both surface and internal target proteins of interest.
Source: VBI Vaccines OxBridge Research, Daily Stock Deals
Important Disclosure: our firm and staff have open positions in TONIX, $TNXP and BioNTech $BNTX
Editor Daily Stock Deals
Get in your inbox FREE insightful analysis that affects your bottom line
learn more.........
Find out the origins of Corona Virus and where it started
Death toll is mounting, are the masks any good?
How to Launder Money? The Chinese Money Laundering Method
Corona Virus Killed an Airline, UK based Flybe
OIL WAR THAT PLUNGED THE WORLD INTO A RECESSION
UNPRECEDENTED ACTION FROM FEDERAL RESERVE
About Daily Stock Deals
Daily Stock Deals helps emerging growth companies reach individual and institutional investors. Daily Stock Deals and its affiliates publish research reports, market analysis and daily stock picks to help investors make informed decisions and achieve their individual investment goals. Our Platform is supported by companies we profile on our network, therefore, our views are neither free of conflict, nor intended as advise to buy/sell any securities and we strongly urge you to read our TOS, Disclaimer/Disclosure and consult with qualified experts. If you would like to get your company featured on Daily Stock Deals network or have any questions, please feel free to contact the editor. This email address is being protected from spambots. You need JavaScript enabled to view it. thanks!





















