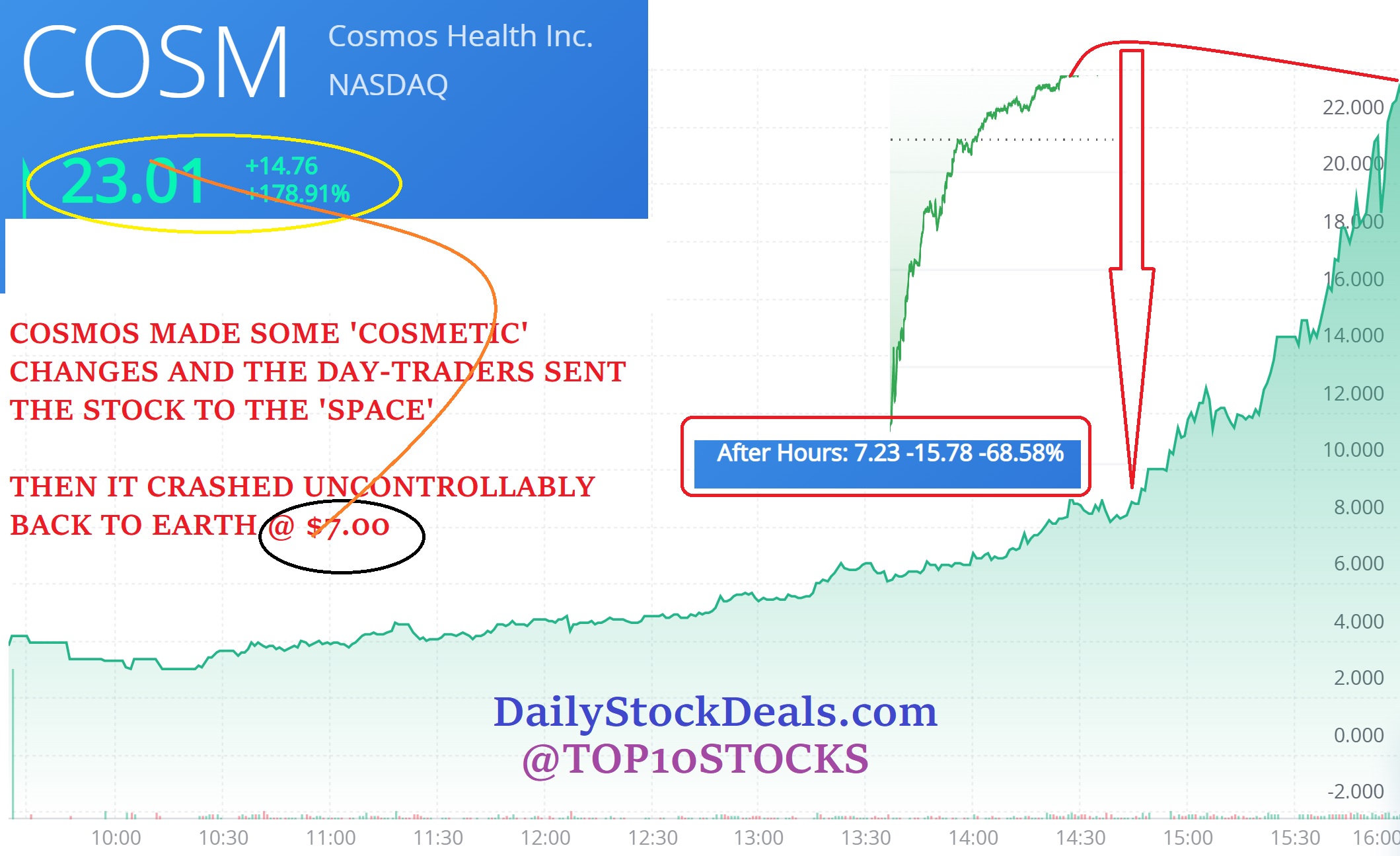
Northwest Biotherapeutics, NWBO, is a clinical stage biotechnology company focused on the development of personalized cancer vaccines designed to treat a broad range of solid tumor cancers.
NW Bio is developing cancer vaccines designed to treat a broad range of solid tumor cancers more effectively than current treatments, and without the side effects of chemotherapy drugs. NW Bio’s proprietary manufacturing technology enables the Company to produce its personalized vaccine in an efficient, cost-effective manner. The Company has a broad platform technology for DCVax dendritic cell-based vaccines. The Company’s lead product, DCVax-L, is currently in a 348-patient Phase III trial for patients with newly diagnosed Glioblastoma multiforme (GBM), the most aggressive and lethal brain cancer. The Company’s second product, DCVax-Direct, is currently in a 60-patient Phase I/II trial for direct injection into all types of inoperable solid tumor cancers. The Company has also conducted a Phase I/II trial with DCVax for late stage ovarian cancer together with the University of Pennsylvania. The Company previously received clearance from the FDA for a 612-patient Phase III trial with its third product, DCVax-Prostate, for late stage prostate cancer.
Dendritic Cell Immunotherapy
Development of effective immune therapies for cancer has long been a goal of the medical and scientific communities. The human immune system is very powerful, and also very complex: it is an “army” with many divisions and many different kinds of weapons. Dendritic cells are the “General” of this immune system “army.” They deliver the “marching orders” to the rest of the immune system agents which tell those agents what to attack.
A diagram of some key agents and weapons of the immune system is set forth below:
 DCVax® is a platform technology that uses activated dendritic cells (the master cells of the immune system), and is designed to reinvigorate and educate the immune system to attack cancers. Unlike conventional cancer drugs, which use one active agent to hit one target on the cancer, DCVax uses many active agents to hit many targets on the cancer.
DCVax® is a platform technology that uses activated dendritic cells (the master cells of the immune system), and is designed to reinvigorate and educate the immune system to attack cancers. Unlike conventional cancer drugs, which use one active agent to hit one target on the cancer, DCVax uses many active agents to hit many targets on the cancer.
We believe that at least three key aspects of the DCVax technology contribute to the positive results (described more fully below) seen in clinical trials to date:
(1) DCVax is designed to mobilize the entire immune system, not just one among the many different categories of immune agents in that overall system.
As described above, DCVax is comprised of activated, educated dendritic cells, and dendritic cells are the master cells of the immune system, that mobilize or help the entire immune system. Some of the prominent cancer drugs today are composed of just one type of antibody — and antibodies themselves are just one type of agent in the overall immune system “army” (see Diagram 1 above). In contrast, the full immune system involves many types of antibodies, and also many other kinds of agents besides antibodies. Similarly, there have been a variety of early immune therapies that failed in the past. These, too, typically involved single agents, such as a single one among the many, many types of immune signaling molecules (e.g., a particular interferon or interleukin), or a single type of agent such as T cells alone, etc. In contrast, dendritic cells mobilize all of these different categories of agents, comprising the whole immune system “army,” in combination with each other and in their natural relationships to each other.
(2) DCVax is designed to target not just one but the full set of biomarkers on the patient’s tumor.
As mentioned above, cancer drugs are typically rifle shots aimed at just one target on a patient’s cancer. However, cancer is a complex and variable disease. Tumor profiles vary among patients with the “same” cancer and also vary as the disease progresses. Further, when rifle shot drugs hit individual targets on cancers, the cancers find ways around them (called “escape variants”) — and the rifle shot treatments then usually stop working. DCVax takes the opposite approach: instead of aiming at a single target, DCVax is designed at to target the full set of biomarkers on a patient’s cancer. Such a treatment approach is expected to make it more difficult for tumors to develop escape variants.
(3) DCVax is personalized, and targets the particular biomarkers expressed on that patient’s tumor.
Extensive scientific evidence has shown that there is substantial variation in tumor profiles and characteristics among patients with the “same” cancer. The degree of variation is particularly enormous in some of the most aggressive cancers, such as GBM brain cancer and pancreatic cancer. Cancer drugs are typically keyed to a single target which is believed to be found on the cancer cells’ surface or in one of the cancer cells’ signaling pathways in a substantial percentage of patients with a given type of cancer. Such drugs can be of no use in patients whose cancers do not happen to express that particular target, or cease expressing that target as the disease progresses. Most cancer drugs only achieve clinical benefits in a limited percentage of the patients with the type of cancer being targeted (e.g., 25 – 30% of the patients). In contrast, DCVax has achieved clinical benefits (i.e., longer delay in disease progression and longer extension of survival than with standard of care treatment) in over 80% of the patients who have received DCVax in clinical trials to date. Since DCVax is made with biomarkers from the patient’s own tumor, it is automatically tailored to targets that are present on that patient’s cancer.
The DCVax technology is expected to be applicable to most cancers, and is embodied in several distinct product lines. One of the product lines (DCVax®-L) is designed to cover all solid tumor cancers in which the tumors can be surgically removed. Another product line (DCVax®-Direct) is designed for all solid tumor cancers which are considered inoperable and cannot be surgically removed. We believe the broad applicability of DCVax to many cancers provides multiple opportunities for commercialization and partnering.
On November 17, 2022 the Company Published the latest Phase 3 results in JAMA
Both Median Survival and “Long Tail” of Extended Survival Were Increased In Both Newly Diagnosed and Recurrent Glioblastoma
Results Featured In JAMA Oncology Peer Reviewed Publication
BETHESDA, Md., November 17, 2022 – Northwest Biotherapeutics (OTCQB: NWBO) (“NW Bio”), a biotechnology company developing DCVax® personalized immune therapies for solid tumor cancers, today reported that in its Phase III clinical trial both median survival and the “long tail” of extended survival were increased in both newly diagnosed and recurrent glioblastoma brain cancer patients treated with DCVax®-L. The trial has met both the primary and the secondary endpoint under the Statistical Analysis Plan for the trial.
The trial results were reported today in a featured publication co-authored by more than 70 physicians from leading institutions across the U.S., Canada, U.K. and Germany, in the peer reviewed cancer journal JAMA Oncology, entitled “Association of Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination with Extension of Survival Among Patients with Newly Diagnosed and Recurrent Glioblastoma”. https://jamanetwork.com/journals/jamaoncology/fullarticle/2798847
The Company believes this is the first time in nearly 20 years that a Phase III trial of a systemic treatment has shown such survival extension in newly diagnosed glioblastoma, and the first time in nearly 30 years that a Phase III trial of any type of treatment has shown such survival extension in recurrent glioblastoma.
Ms. Powers, CEO of NW Bio, commented: “We are excited to see the meaningful survival extensions in glioblastoma patients treated with DCVax®-L in this trial – particularly in the “long tail” of the survival curve, where we see more than double the survival rates as with existing standard of care. With well over 400 clinical trials for glioblastoma having failed over the last 15 years, it is gratifying to be able to offer new hope to patients who face this devastating disease.”
Glioblastoma is the most common and most lethal form of primary brain cancer. Standard of care (SOC) treatments have been virtually unchanged for nearly 20 years. With SOC treatments, patients typically survive for only about 15-17 months from diagnosis, with the tumor recurring at about 6-8 months from diagnosis and the patients typically surviving for about 7-9 months after recurrence. Five-year survival from diagnosis is only about 5%.
Daily Stock Deals is an affiliated/partner property, please read TOS/Disclaimer/Disclosure, thanks!
Editor, Daily Stock Deals
Keep an eye on this space for major announcements!
Reminder: Free Membership enables immediate access to @TOP10STOCKS a great way to start your trading day
Source: The Company, OxBridge Research, Daily Stock Deals, PennyStockIQ