Oncotelic Therapeutics, OTLC, is a unique Immuno-Oncology company at the cutting edge of medical research and discovery of precision medicine – Oncotelic is creating new pathways by combining AI tools to accelerate discovery by a magnitude, the AI (in the cloud) helps their team of scientists select the best and most effective molecules, eliminate subpar molecules from the earliest stage of discovery, thereby, saving precious time and increasing the chances of breakthrough discoveries, eliminating the probability of errors and omission by a significant margin, and by merging the AI tools with proven expertise in medicine, the Oncotelic team has cut the time and reduced R&D budget leakage and waste by a huge margin, at Oncotelic every R&D dollar is optimized to deliver better ROI and highly effective drugs/therapies in the most efficient manner than ever before.
Oncotelic is led by one of the most renowned scientists in America, a pioneer in immuno-oncology, a man with 39 Patents to his name and over 100 Patent Applications Pending – Dr. Vuong Trieu.
Dr. Vuong was a joint patent holder who helped develop the block-buster billion dollar drug Abraxane™ – now owned by Bristol-Myers Squibb, NYSE: BMY, a $144 billion dollar company.
Dr. Vuong has put together a team of highly distinguished scientists and researchers, and a superb management team with years of proven marketing, brand building and scaling experience – a team that has turned startups into multi-billion dollar enterprises.
Oncotelic Developing Distinct Drugs Targeting Several Extremely Brutal Diseases (Each of them has the potential to be a block buster) let’s focus on one for now.
Primary Focus:
The one we are most excited about for the time being (without diminishing the importance of the other drugs that are in various stages of development) is a potential therapy that could increase the efficacy of an existing FDA approved drug (by as much as 100%) a drug that has generated over $17 billion in revenue last year alone and expected to hit $20 billion in 2025. The name of the drug is Keytruda owned by Merck.
Keytruda meet > OT-101
What Dr. Vuong and his excellent team did here is absolutely breathtaking! Dr. Vuong likes Keytruda, it’s great and it’s helping a lot of folks but it could do more, a whole lot more! When you combine the existing drug (Keytruda) with Dr. Vuong’s breakthrough discovery (after the FDA approval) the initial indications are that the rate of efficacy of Keytruda can increase by up to 100%!
Dr. Vuong’s newly discovered – inhibitor blocker eliminator – when given to patients along with Keytruda, the patients who are currently not benefiting can benefit! Imagine the relief combo-therapy can bring to the patients and their families around the world, right now, a lot of patients who can not benefit from Keytruda alone – can soon benefit from the combination therapy which Dr. Vuong has discovered!
Dr. Voung’s discovery is one of the most brilliant things I’ve ever seen – let me recap – A highly successful FDA approved Block Buster Drug that’s on the market today, whose efficacy can jump as much as 100% from where it is now – That’s what motivates me and the team at Oncotelic, a lot of patients and their families are needlessly suffering now!
As an investor you obviously look at the size of addressable market and that’s what motivates investors, right? As I’ve mentioned Keytruda has made $17 billion for Merck last year – if Dr. Vuong’s invention/discovery helps increase the efficacy rate of Keytruda even by only 50%, you do the math! We are talking about an opportunity worth billions! Think about it.
OT-101 Pipeline
Oncotelic is an artificial intelligence driven immuno-oncology company with a robust pipeline of first in class TGF-β immunotherapies for late stage cancers such as gliomas, pancreatic cancer and melanoma. OT-101, the lead immuno-oncology drug candidate of Oncotelic, is a first-in-class anti-TGF-β RNA therapeutic that exhibited single agent activity in relapsed/refractory cancer patients.
Rare Pediatric Cancer Designation
Oncotelic is seeking to leverage its deep expertise in oncology drug development to improve treatment outcomes and survival of cancer patients with a special emphasis on rare pediatric cancers. Oncotelic also has rare pediatric designation for DIPG (OT-101), melanoma (CA4P), and AML (OXi 4503). The Company also acquired (“PointR”) Data in November 2019.
Management Team
Dr. Voung Trieu, PHD, CEO, Chairman
Dr. Trieu, an expert in pharmaceutical development, currently serves as CEO/Chairman of Oncotelic Inc. Previously he was President and CEO of Igdrasol- developer of 2nd generation Abraxane- where he pioneer the regulatory pathway for approval of paclitaxel nanomedicine through a single bioequivalence trial against Abraxane. When Igdrasol merged with Sorrento Therapeutics, he became CSO and Board Director. He was Board Director of Cenomed- a company focusing on CNS drug development. Before that he was Director of Pharmacology, Pharmacokinetics, and Biology at Abraxis where he lead the development of albumin encapsulated therapeutics along building high throughput platform for small molecules, mirRNA, kinases. Prior to that he was Group Leader at Applied Molecular Evoluton where he was developing biobetter for Humira and Enbrel. Before that he was Director of Cardiovascular Biology at Parker Hughes Institute. Dr. Trieu holds a PhD in Microbiology, BS in Microbiology and Botany. He is member of ENDO, ASCO, AACR, and many other professional organization. Dr. Trieu published widely in oncology, cardiovascular, and drug development. Dr. Trieu has over 100 patent applications and 39 issued US patents.
Seymour Fein, MD, CMO
Dr. Fein’s professional activities have been focused on drug development research for over 35 years. He has been extensively involved in the successful evelopment of numerous drugs, biologics and medical devices over this time leading to FDA approvals for over 20 drugs (NDAs, sNDAs, BLAs) and devices (PMAs). Dr. Fein began his career at Hoffmann-La Roche Ltd. as a senior research physician and was responsible for a clinical development program that led to U.S. Food and Drug Administration (FDA) approval of recombinant interferon-alpha for cancer treatment. Dr. Fein was also the medical director of Bayer Healthcare Pharmaceuticals (U.S.) where he was responsible for therapeutic areas including gastroenterology, oncology, and cardiology. He later served as medical director for Rorer Group (now part of Sanofi) and Ohmeda (now part of Baxter). Dr. Fein founded and has been managing partner of a clinical and regulatory consulting organization and has worked closely with the Division of Gastroenterology and Inborn Errors Products at the FDA. He has participated in the development of and FDA approval of numerous drug products in many therapeutic areas. Dr. Fein has successfully overseen entrepreneurial drug development leading to the FDA approval of two orphan drug products in the field of gastroenterology.
Dr. Fein received his B.A. degree from the University of Pennsylvania and his M.D. degree with honors from New York Medical College. He completed a three-year residency in internal medicine at Dartmouth and a three-year fellowship in medical oncology and hematology at Harvard Medical School, where he served as an instructor of medicine during his final fellowship year. Dr. Fein is board-certified in both oncology and internal medicine.
Amit Shah, CFO
Amit Shah, age 53, has served as a senior financial officer for a number of life science companies, including Chief Financial Officer at Marina Biotech, Inc., a publicly traded biotechnology company (2017 to 2018); Vice President of Finance & Accounting and Acting Chief Financial Officer at Insightra Medical Inc. (2014 to 2015); VP Finance and Acting Chief Financial Officer at IgDraSol Inc. (2013); Corporate Controller & Director of Finance at ISTA Pharmaceuticals (2010 to 2012); Corporate Controller at Spectrum Pharmaceuticals (2007 to 2010): and as Controller / Senior Manager Internal Audits at Caraco Pharmaceuticals Laboratories (2000 to 2007). In addition to his work with life sciences companies, Mr. Shah served as the Chief Financial Officer at Eagle Business Performance Services, a management consulting and business advisory firm (2018 through March 2019) and as a consultant and ultimately Senior Director of Finance – ERP, at Young’s Market Company (2015 to 2017). Mr. Shah received a Bachelor’s of Commerce degree from the University of Mumbai, and is an Associate Chartered Accountant from The Institute of Chartered Accountants of India. Mr. Shah is also an inactive CPA from Colorado, USA
Saran Saund, CBO/GM of AI Division
Silicon Valley entrepreneur, Saran has been founder, CEO and GM at startups and public companies. Passionate about applying technology innovations to real world markets, he successfully founded an AI consortium to accelerate enterprise adoption of AI which engaged leading universities and technology vendors. A startup veteran, his track record includes senior leadership roles at companies that were acquired by leaders such as Marvell (MRVL) and Qualcomm (QCOM). His startup Cybercash (CYCH) had a successful IPO on NASDAQ. Saran started his career at Xerox PARC pushing 1’s and 0’s as a software engineer
Anthony E. Maida III, PhD, Chief Clinical Officer-Translational Medicine
Dr. Maida, an expert in immuno-oncology, currently serves as Senior Vice President – Clinical Research at Northwest Biotherapeutics, Inc. Prior to joining Northwest Dr. Maida served as Vice President, Clinical Research and General Manager, Oncology, World-wide at PharmaNet, Inc. Prior to joining PharmaNet Dr. Maida served as Chairman, Founder and Director of BioConsul Drug Development Corporation and Principal of Anthony Maida Consulting International, servicing pharmaceutical firms, venture capital, hedge funds and Wall Street. Dr. Maida’s skill set includes the leading execution and oversight of finance, operations, research, clinical and scientific development, regulatory and manufacturing for the development of various oncology immunotherapies. Over the past 25 years Dr. Maida has served in a number of executive roles, including, Chairman, CEO, COO, CSO, CFO and business development. Over recent years Dr. Maida has raised, or assisted in financings, nearly $200 million for emerging biotechnology companies. Dr. Maida serves as an advisor, consultant and technical analyst for CMX Capital, LLC, Sagamore Bioventures, Roaring Fork Capital, Toucan Capital, North Sound Capital, The Bonnie J. Addario Lung Cancer Foundation and vFinance; the later three companies are located on the East Coast. Additionally, Dr. Maida has been retained by Abraxis BioScience, Inc., Northwest BioTherapeutics, Inc. and Takeda Chemical Industries, Ltd. (Osaka, Japan). Dr. Maida holds a Ph.D. in Immunology, a B.A. degree in Biology, a B.A. Degree in History, a MBA and a MA in toxicology. He is a member of the American Society of Clinical Oncology (ASCO), the American Association for Cancer Research
About Daily Stock Deals
Daily Stock Deals helps emerging growth companies reach individual and institutional investors. Daily Stock Deals and its affiliates publish research reports, market analysis and daily stock picks to help investors make informed decisions and achieve their individual investment goals. Our Platform is supported by companies we profile on our network, therefore, our views are neither free of conflict, nor intended as advise to buy/sell any securities and we strongly urge you to read our TOS, Disclaimer/Disclosure and consult with qualified experts. If you would like to get your company featured on Daily Stock Deals network or have any questions, please feel free to contact the editor. editor@DailyStockDeals.com thanks!
Daily Stock Deals is an affiliated/partner property, please read TOS/Disclaimer/Disclosure, thanks!
=====—–=====—-=-=-=-=-=-=-=-=-=-=====——-===-=-=-===-=-======——====-=-=

Genprex, GNPX, Profile, Summary
Genprex, GNPX, is a clinical stage gene therapy company pioneering a new approach to treating cancer. The company is developing gene technologies based on a novel proprietary technology platform, their first product candidate is called Oncoprex™ immunogene therapy, or Oncoprex, their platform technologies are designed to administer cancer fighting genes by encapsulating them into nanoscale hollow spheres called nanovesicles. The nanovesicles are then administered intravenously and are taken up by tumor cells where they express proteins that are missing or found in low quantities.
Oncoprex™, targets non-small cell lung cancer. This initial cancer target represents about 85 percent of all lung cancers and causes more deaths each year than any other type of cancer. Lung cancer is the second most common form of cancer, and the five-year survival rate for late stage lung cancer has not improved substantially in a quarter century. Oncroprex may be used alone or in combination with other cancer therapies, to combat additional types of cancer.
Fast Track

On January 14, 2020, the Company received U.S Food and Drug Administration (FDA) Fast Track Designation for its Oncoprex immunogene therapy in combination with the EGFR tyrosine kinase inhibitor (TKI) osimertinib (AstraZeneca’s Tagrisso®, which had worldwide sales in 2018 of $1.86 billion and $2.31 billion in the first nine months of 2019, and it is currently AstraZeneca’s highest grossing product) for the treatment of NSCLC patients with EFGR mutations that progressed after treatment with osimertinib alone. Oncoprex consists of the TUSC2 (Tumor Suppressor Candidate 2) gene, the active agent in Oncoprex, complexed with a lipid nanovesicle.
The Company’s current Phase I/II clinical trial utilizes the combination of the EGFR inhibitor erlotinib (marketed by Genentech in the U.S. and elsewhere by Roche as Tarceva®) and Oncoprex against NSCLC. The current Phase I/II trial is active but is not currently enrolling patients, though the Company had planned to resume enrollment in mid-2020. Tumor shrinkage in patients resistant to erlotinib enrolled in this trial showed that Oncoprex can overcome resistance to TKIs and provided support for the Fast Track Designation. Osimertinib is now considered a new standard of care for NSCLC patients with an EGFR mutation. Given this and receipt of FDA’s Fast Track Designation for use of Oncoprex combined with osimertinib in patients whose tumors progress on osimertinib, the Company has decided to prioritize this drug combination and patient population. Therefore, the Company plans to initiate a Phase I/II clinical trial of Oncoprex combined with osimertinib in mid-2020 at multiple cancer centers across the United States, and it does not intend to reopen enrollment in the current Phase I/II trial using the combination of Oncoprex and erlotinib at this time.
Genprex plans to file an amendment to its Investigational New Drug (IND) application with the FDA for the Oncoprex and osimertinib combination therapy trial within the first quarter of 2020. Upon FDA acceptance of the amendment, the Company expects to be in a position to enroll patients shortly thereafter. The Company believes that enrollment in the new clinical trial may be rapid, as the tumors of almost all patients who are treated with osimertinib progress after treatment, and these patients may be candidates for its clinical trial combining Oncoprex with osimertinib, for which the Company received Fast Track Designation.
The Company believes prioritizing the combination therapy of Oncoprex with osimertinib represents the most efficient way to advance its lead drug candidate through the clinical process for FDA approval, to have the best commercial success in the $17.9 billion global lung cancer market, the Company will also proceed with its plan to file an IND for the additional combination therapy of Oncoprex combined with the immunotherapy drug pembrolizumab (marketed as Keytruda® by Merck in the U.S.) for NSCLC.
Genprex and University of Pittsburgh Sign Exclusive License Agreement for Gene Therapy Candidate for Diabetes
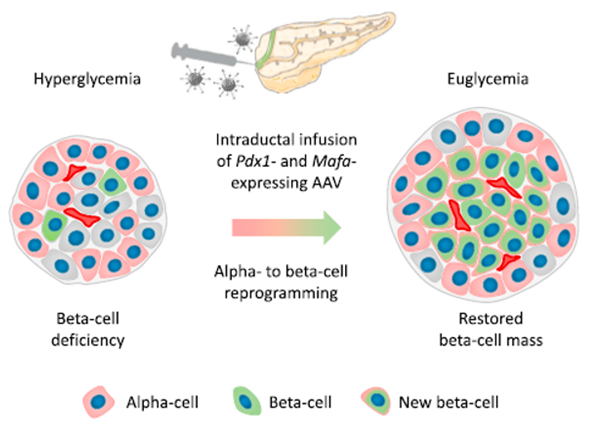
Genprex recently signed an exclusive license agreement with the University of Pittsburgh for a diabetes gene therapy that may have the potential to cure Type 1 and Type 2 diabetes.The diabetes gene therapy, which was developed by lead researcher and Harvard graduate, Dr. George Gittes, at the Rangos Research Center at UPMC Children’s Hospital of Pittsburgh, works by reprogramming beta cells in the pancreas to restore their function, thereby replenishing levels of insulin. The novel infusion process uses an endoscope and an adeno-associated virus (AAV) vector to deliver Pdx1 and MafA genes to the pancreas. The proteins these genes express transform alpha cells in the pancreas into functional beta-like cells, which can produce insulin but are distinct enough from beta cells to evade the body’s immune system.
The diabetes gene therapy has been tested in vivo in mice and nonhuman primates. In studies of diabetic mice, the gene therapy approach restored normal blood glucose levels for an extended period of time, typically around four months. According to Dr. Gittes, the duration of restored blood glucose levels in mice could translate to decades in humans. Following preclinical studies, Dr. Gittes and his team plan to begin a Phase I clinical trial in diabetic patients, which could be the first-ever gene therapy tested in humans for diabetes.
Genprex’s licensed diabetes gene therapy technology works to reprogram alpha cells in the pancreas into beta-like cells, restoring their function, thereby replenishing levels of insulin.
Image source: Osipovich, Anna & Magnuson, Mark. (2018). Alpha to Beta Cell Reprogramming: Stepping toward a New Treatment for Diabetes. Cell Stem Cell. 22. 12-13. 10.1016/j.stem.2017.12.012.
According to the American Diabetes Association, more than 30 million Americans have diabetes, and approximately 1.5 million Americans are diagnosed with diabetes every year. Diabetes patients have the continuous burden of checking and monitoring their blood glucose levels and injecting insulin on a daily basis. Without effective management of diabetes, patients are at risk of stroke, hyperglycemia, cardiovascular disease, diabetic ketoacidosis and extremity amputation. Diabetes is the seventh leading cause of death in the U.S.
Source: Genprex, OxBridge Research, Daily Stock Deals
About Daily Stock Deals
Daily Stock Deals helps emerging growth companies reach individual and institutional investors. Daily Stock Deals and its affiliates publish research reports, market analysis and daily stock picks to help investors make informed decisions and achieve their individual investment goals. Our Platform is supported by companies we profile on our network, therefore, our views are neither free of conflict, nor intended as advise to buy/sell any securities and we strongly urge you to read our TOS, Disclaimer/Disclosure and consult with qualified experts. If you would like to get your company featured on Daily Stock Deals network or have any questions, please feel free to contact the editor. editor@DailyStockDeals.com thanks!
If you would like to get your company featured on OxBridge Research or like to learn more, please contact the editor. Thank you!
=-=-=-=-=-=-=-===–===-=-=-=-=–=-=-=-=–=-=-=-=-=-=-=-=-=-=-=-=-==-=-=-=-=-=-=-==

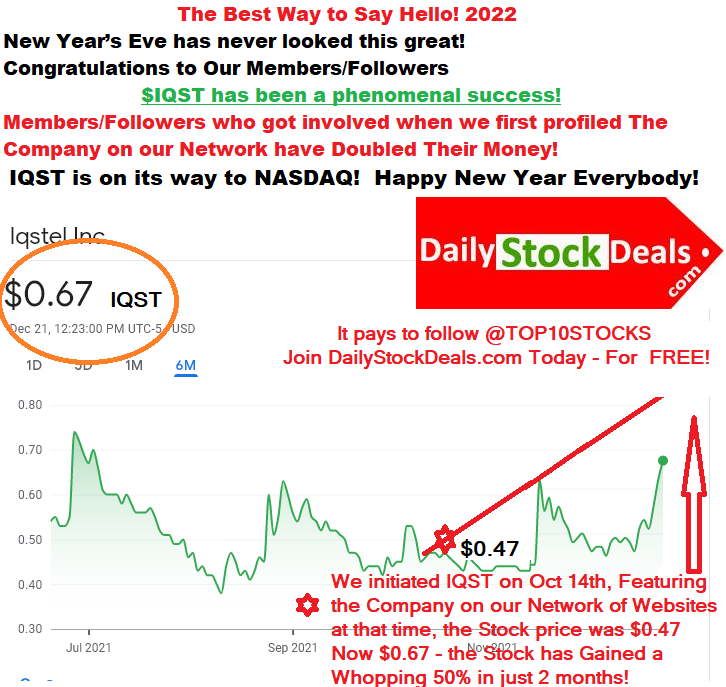
iQSTEL, IQST, Company Profile, Summary
iQSTEL, IQST, is based in Florida and serves mainly countries in the Caribbean and Latin America, the region of Latin America and the Caribbean is one of the fastest growing consumer markets in the world.
The company’s top executives have a great insight and deep knowledge of the region, its people and know how the governments in these countries operate. The unique insight has helped the company establish successful telecom, transport and financial services in each country then they manage to interconnect consumers from their neighboring countries, opening up more opportunities for people to communicate and trade and loop back to the USA, making the network as a whole more valuable and scalable. iQSTEL has created a vibrant ecosystem that has been growing each year, contributing double digit revenue gains and helping company introduce new services and gain more customers.
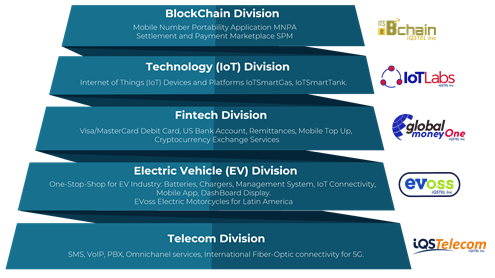
Telecom
Services: SMS, VoIP, PBX, Omnichanel services, International Fiber-Optic connectivity for 5G.
Electric Vehicle (EV)
Services: Electric Motorcycles for Latin America, One – Stop – Shop solutions for Electric Vehicles (EV) industry: EV Batteries, EV Chargers, EV Battery Management System, IoT Connectivity, Mobile App for EV connectivity, EV Dashboard Display, etc.
Fintech
Services: Visa/Mastercard Money One (Visa/Mastercard debit card), Remittances, Mobile Top Up, Cryptocurrencies Exchange Services.
Technology (IoT)
Services: Internet of Things (IoT) Devices and Platforms IoTSmartGas, IoTSmartTank.
Blockchain
Services: Blockchain Platforms Solutions: Mobile Number Portability Application (MNPA), Settlement and Payment Marketplace (SPM).
About Daily Stock Deals
Daily Stock Deals helps emerging growth companies reach individual and institutional investors. Daily Stock Deals and its affiliates publish research reports, market analysis and daily stock picks to help investors make informed decisions and achieve their individual investment goals. Our Platform is supported by companies we profile on our network, therefore, our views are neither free of conflict, nor intended as advise to buy/sell any securities and we strongly urge you to read our TOS, Disclaimer/Disclosure and consult with qualified experts. If you would like to get your company featured on Daily Stock Deals network or have any questions, please feel free to contact the editor. editor@DailyStockDeals.com thanks!
Daily Stock Deals is an affiliated/partner property, please read TOS/Disclaimer/Disclosure, thanks!
-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-
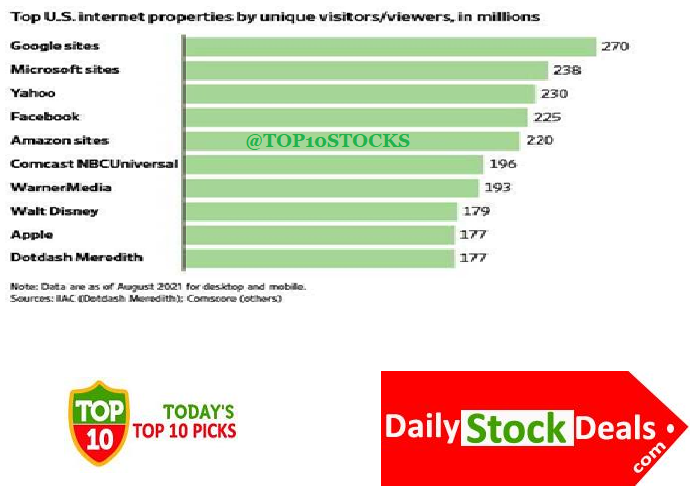
Top 10 Most Visited US Websites, Top 10 US Portals
=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-==-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=
DigitalTown, DGTW, Company Profile, Business Summary
DigitalTown, DGTW, Corporate Profile
DigitalTown (DGTW), a fully reporting publicly traded company currently listed on the OTC Markets, however, the company has plans in place to up-list on NASDAQ/NYSE in the near future.
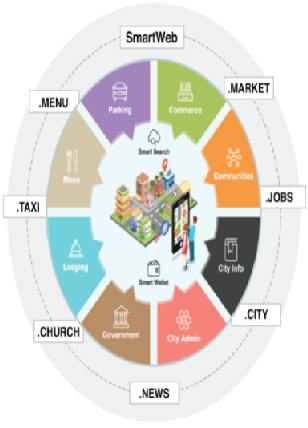
DigtalTown is a unique company partnering with city governments to build strong, resilient communities and flourishing local businesses.
DigitalTown provides cities a highly scalable, digital infrastructure that helps cities connect with citizens and local businesses with ease. The DigitalTown powered, mobile friendly, city portal empowers citizens to interact directly with city leaders, local government agencies and businesses.
Act Local Think Global
DigitalTown changing the landscape one city at a time, helping build smart cities and better governments. City governments eagerly embracing DigitalTown’s vision because it helps cities provide better services quickly and cost effectively. DigitalTown’s has a unique ability to capture big-data at a very granular level, the unprecedented access to information helping city mayors and administrators plan better cities, better communities and optimize the use of city resources.
Changing Dynamics of Local Commerce
Partnering with DigitalTown helps cities collect taxes and fees efficiently and cost effectively, and helps local businesses sell more products and services to people in their own communities. Research shows that people want to patronize local businesses, unfortunately, they don’t have easy access to buy local. DigitalTown changing the dynamics of local commerce, empowering cities and citizens like never before.
Helping Cities and Citizens
DigitalTown powered city portal helps citizens and businesses pay their utility bills, property and business taxes and buy and sell goods and services without leaving the city portal. The portal acts like town square, bustling with commerce, increasing social interaction and community engagement. The company believes that this will help increase standard of living, reduce crime, increase property value and attract more people and businesses to DigitalTown powered cities.
Highly Scalable, Cloud Based Platform
DigitalTown’s highly scalable, cloud based platform built for highly interconnected, extremely dynamic and mobile friendly cities of the future. Digital Town bringing people together and changing the landscape of cities and towns.
Why Invest in DigitalTown?
DigitalTown is a unique, high growth, extremely scalable SaaS platform, and it has many streams of income. The company generates revenues through licensing fees, software development and integrations fees, payment processing fees, native ads and sponsored content and other sources.
DigitalTown powered city portals are social and commerce hubs, consumers recommend their favorite restaurants & beauty shops, write reviews, discover local events, find doctors, dentists and plumbers. The portal helps peer-to-peer and consumers-to-business communication, helping consumers connect with each other and shop local.
DigitalTown provides a one stop shopping for all the goods and services from a single platform. DigitalTown helps cities and business process transactions and facilitates safe and secure payments. Local businesses could easily integrate and/or build their own digital stores. Research shows that most consumers for personal data security reasons are reluctant to shop on obscure, small store websites, DigitalTown helps alleviate the concerns and encourages people to shop at their favorite local stores. Every transaction on DigitalTown is end-to-end encrypted, therefore, your personal and payment information stays secure and out of the reach of data thieves.
DigitalTown Growing Rapidly
The company is growing organically and through smart acquisitions. The company acquired and successfully integrated half a dozen companies and looking to acquire more as it continues to grow at a rapid pace.
Recently acquired companies
The Management Team
The DigitalTown team is led by successful technology managers who have a passion for economic development and fostering robust local economies.
Robert W. Monster
CEO & President
Robert joined DigitalTown at CEO in 2015, bringing significant experience with him. Among his many accomplishments, he founded and served as Managing Director of Monster Venture Partners LLC, and founded Global Market Institute (GMI). Prior to founding GMI, he was a global product development manager at Procter & Gamble. Robert earned both a BS and an MBA from Cornell University. He was recognized as Ernst & Young’s 2006 Entrepreneur of the Year. He also authored Market Research in the Internet Age, published by John Wiley and Sons.
Chris Maxwell
Chief Technical Officer
Chris leads DigitalTown’s development team. He’s responsible for engaging with clients to understand their challenges and needs, and delivering customized solutions to meet them. Prior to joining DigitalTown, he founded Cloud.Market, an online marketplace serving local communities. He was a senior technical program manager at Amazon, CTO of Voxeo Labs (acquired by Aspect), and held leadership and technical roles at Tellme (acquired by Microsoft), AT&T, Edify, Intervoice (acquired by Convergys), Verizon and EDS. Chris earned a BA from Baylor University and an MBA from the University of Dallas.
Adee Wada
Vice President, Marketing
Adee leads DigitalTown’s marketing efforts. He comes from Microsoft, where he spent eight years serving in their Online Media Business division, most recently as Director of Audience Marketing. Prior to Microsoft, he was the Director of Event Services for the Seattle Mariners, overseeing operations at Safeco Field. He earned a BA from the University of Colorado. Adee enjoys life in Bellevue with his wife and three children.
Ken Cooper
Vice President, Finance
Ken brings years of financial experience to DigitalTown, founding and directing Four Hills Advisors. Prior to Four Hills, he spent 14 years at Life Time Fitness, Inc., serving in many roles, helping them grow from $53 million in revenues to over $1 billion. He led their investor-relations efforts from Life Time’s IPO in 2004 thru 2010. He helped garner over $1 billion in capital for the company, and built their blueprint for strategic decisions for years to come. He also led their Athletic Events & Endurance division, as well as serving in their M&A department.
Ken Jensen
Vice President, Engineering
For 20 years, Ken led Software Masters. Acquired by DigitalTown, the company developed website solutions for communities and local governments. Since coming on board, he has taken primary responsibility for the development of software solutions for local governments.
Faris Oweis
Vice President, Corporate Development
Faris has experience in countless industry verticals from tech to architecture. He’s a storyteller, listener, strategist, rapid learner, and a natural connector of ideas who has secured projects across 8 industries in over 30 countries. Prior to DigitalTown, Faris led large pursuits for CH2M with a focus on mega infrastructure and smart city projects across the Middle East and India. School wise he holds a B.S. in Marketing from Virginia Tech (Go Hokies) and MBA from Auburn (War Eagle).
Clint Skidmore
Vice President, Product Development
Since 2001, Clint has been fuelled by a passion for combining technology and travel into a market-leading software solution for the travel industry. As CEO of Rezserve Technologies Ltd, Clint has brought his vision to life, enriching yet simplifying the user experience of booking lodging and other travel related products. Since coming on board at DigitalTown through the acquisition of Rezserve, Clint has led product development and use his experience and expertise to increase the usability and functionality of DigitalTown with an emphasis on providing intuitive Merchant Solutions that help businesses of all sizes to win locally and compete globally.
Ala Dadan
Vice President, Product Design
With over 14 years of experience in new media design. Ala Dadan leads the design efforts behind the new product features in DigitalTown.com across their full life cycle — concept, scope, design comps, and prototype support. He started his career in 2001 & during that time he was a member of different design teams in several companies in Jordan – the Middle East and as a consultant for many design firms abroad. Mr. Dadan has also served as Vice President of Design at Epik.com and was the founder of O2 Alternative, a trend-setting design house in Jordan.
Kenneth A. Holloway
Marketing Director
Kenny joined us in March 2016. As Marketing Director, Kenny oversees the knowledge base, training, support and SEO. Prior to DigitalTown, Kenny had spent over a decade at the helm of 360 Media Group. Kenny brings a wide range of experience with media buys, traffic generation, web development and is a published photographer.
Please visit the company website DigitilTown.com and learn how DigitalTown helping local communities. Make your city a – DigitalTown Empowered City – today!
Don’t miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!® Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks. If you want to learn more or get your company featured on Daily Stock Deal, please contact the Editor. editor [@] DailyStockDeals.com