In October 2019, Biogen, BIIB, stunned the medical community by announcing that it will seek FDA approval for a drug that it had discarded more than six month ago. The company and its partner, Eisai, at that time said that the drug failed to significantly improve cognitive functions in Alzheimer’s patients.
Why change of heart?
The company now claims that they ‘overlooked’ some critical data and a more closer analysis of the data revealed that a group of trial participants who were given a larger doses of the drug had a significant improvement in cognitive functions, the company said that it has submitted the newly discovered data to the FDA and is seeking approval to market the drug.
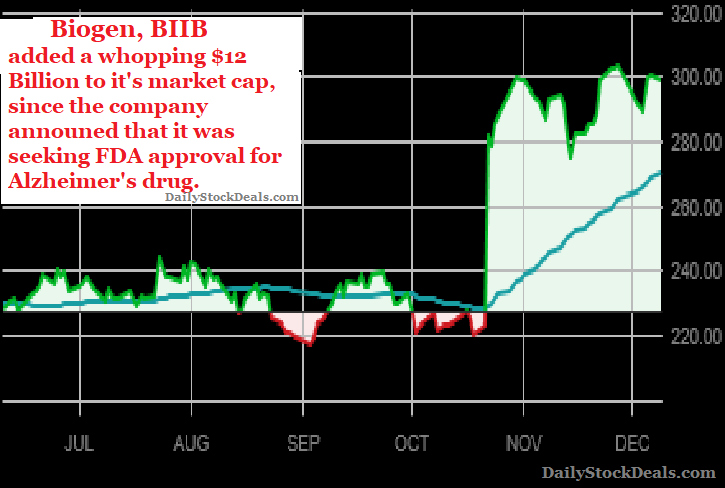
The announcement unleashed a stock buying frenzy that added a whopping $12 billion to the company’s market cap, remember, the drug has not been approved yet!
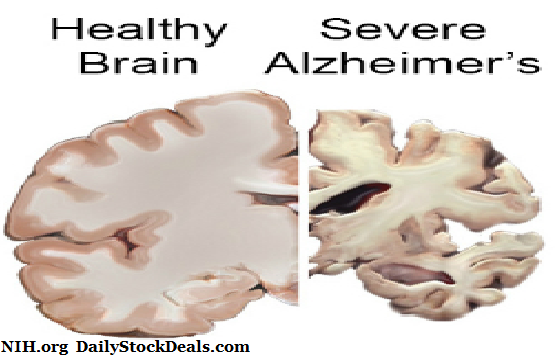
More than 6 million Americans are suffering from Alzheimer’s and there is no drug to stop it, so the company is betting that the FDA may approve the drug despite the treatment only marginally improves the quality of life. Alzheimer’s is a brutal disease that slowly takes away the ability to live and look after oneself, most patients suffer severe dementia and ultimate death.
Editor Daily Stock Deals
Gain Insight, Trade with Confidence, Join Fellow Investors, click now
Source: NIH, CDC, OxBridge Research, Daily Stock Deals
TOP 10 E-Cigarettes and Vaping Facts you should know!

